Laboratory of Thermophysical Properties
|
Department: Department of Thermodynamics Head: Ing. Václav Vinš, Ph.D., DSc. |
|
Laboratory of Thermophysical Properties focuses on description of thermodynamic properties of fluids including pressure-temperature-composition-density relationships and vapor-liquid-solid phase equilibria and transport properties such as viscosity and surface tension. Accurate description of these properties can significantly improve design, efficiency and reliability of various technical applications including cooling and refrigeration, processes in power industry or treatment of waste CO2-rich mixtures in CCS/U (carbon capture and storage/utilization). The Laboratory develops and implements precise methods for the measurement and modeling of thermophysical properties of various technically relevant fluids such as heat transfer liquids, alternative refrigerants, ionic liquids, and reference fluids and standards of thermophysical properties. The Laboratory develops correlations and models of fluid properties intended for use in industry, research and development. The modeling approaches cover semi-empirical equations of state, group contribution methods and molecular dynamics simulations.
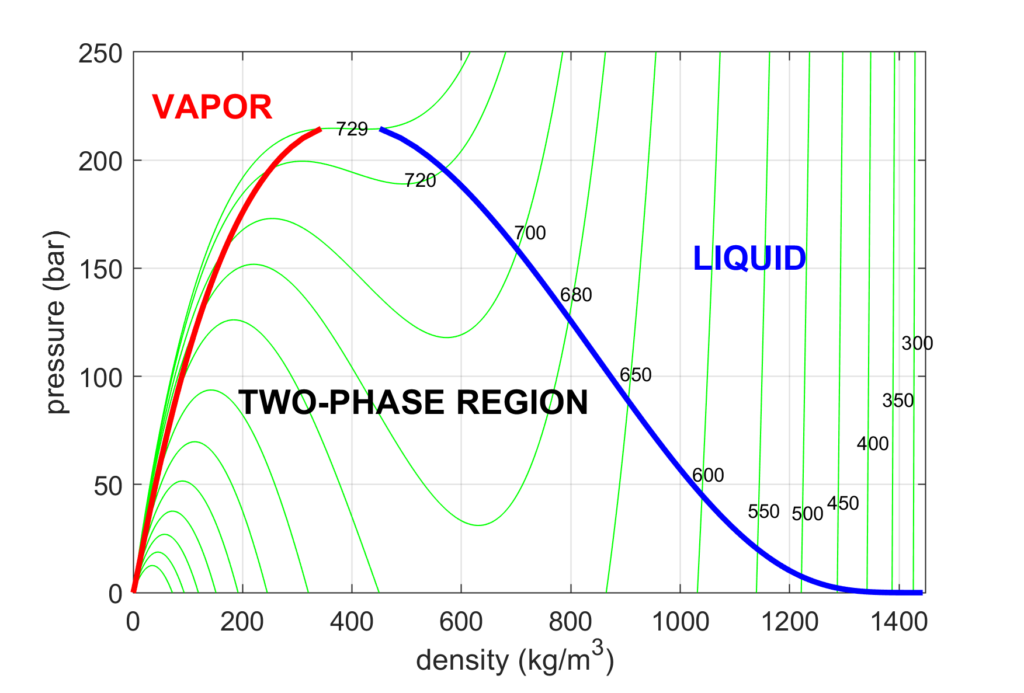
Vapor-liquid phase diagram of pure H2O2
|
Head: Dr. Václav Vinš, Res.Prof.
Researchers:
Dr. Miroslav Čenský
Dr. Olga Prokopová
Dr. Monika Součková
Postdocs & Ph.D. candidates:
Dr. David Celný
Dr. Jaroslav Pulec
Former members:
Dr. Ali Aminian
Jaroslav Klomfar MSc.
Dr. Jiří Hykl
Dr. Jaroslav Pátek (former head, retired)
Students:
Nicolas Hénin (Centrale Méditerranée), 2025 – 6 months internship
Gabriela Spilková (FJFI CTU in Prague), 2022 – Bachelor student
Aleksandr Vasilev, MSc. (Technische Universität Dresden), 2021 – 1.5 months internship
Josefine Gatter (Technische Universität Dresden), 2019 – 3 months internship
Laboratory of Thermophysical Properties (LTP) carries out experimental and theoretical research in the field of thermodynamic and transport properties of fluids. Investigated systems include aqueous mixtures, modern dielectric heat transfer fluids, alternative refrigerants, ionic liquids, and water + gas mixtures forming gas hydrates. The motivation is to provide highly-accurate experimental data that can be used as reference both in research and technical applications. The experimental measurements are carried out using unique apparatuses of own design together with commercial instruments. Advanced calibration procedures are developed in order to obtain well-defined and reliable uncertainty estimates of the measured data. The experiments are accompanied by theoretical research where the semi-empirical models such as multiparameter or SAFT-type equations of state together with the molecular dynamics tools are employed.
>Density, surface tension and viscosity of technical fluids
The experimental research involves accurate measurements of pressure-volume-temperature-composition relationships, surface tension and viscosity of various technical fluids using carefully operated commercial instruments. Temperature-composition dependence of density at atmospheric pressure is measured using a vibrating tube densimeter Anton Paar DMA 5000 M. A high pressure vibrating tube instrument Anton Paar DMA HP equipped with an in-house developed pressure setup and other accessories provides density and compressibility data in the temperature range from -10 °C to 200 °C and pressures up to 70 MPa. Both instruments operate on the principle of a vibrating U-tube, whose characteristic (oscillation) frequency depends on the density of liquid sample filled inside the U-tube. The surface tension is determined with the tensiometer Krüss K100 Mk2 using the Wilhelmy plate method and the du Noüy ring technique in the temperature range from -15 to 90 °C. In addition, a buoyancy density measurement at atmospheric pressure with a single-crystal silicon can be carried out for samples with density up to 2000 kg/m3. The advantage of comparative buoyancy measurements is that, unlike in case of vibrating densimeters, the determined density does not depend on the viscosity of the sample. A recently acquired Stabinger viscometer Anton Paar SVM 3001 allowing for measurement of kinematic viscosity and additionally also density at temperatures from -60 °C to 135 °C is currently brought into operation.
Surface tension of aqueous systems
Under the collaboration with the International Association of Properties of Water and Steam IAPWS, the LTP team investigates properties of aqueous systems including water mixtures with alcohols and salts. These systems exhibit scientifically interesting behavior, whose better description is important in technical applications and environmental studies. The Laboratory focuses mainly on lower temperatures including the metastable supercooled state, when a small amount of liquid can stay for a short time at temperatures even several tens of degrees below the equilibrium freezing point.
Together with the Laboratory of Phase Transitions the LTP team operates an in-house developed apparatus for the measurement of surface tension under the metastable supercooled state. The apparatus helps to obtain unique data for the surface tension of aqueous systems at temperatures between -30 °C and +50 °C. Main aim of the measurements is to help to clarify some of the anomalies of the cold and supercooled water, e.g., the second inflection point (SIP) in the temperature trend of the surface tension of water detected by previous experiments and some molecular simulations. The measuring technique is based on the capillary elevation in a fused silica capillary tube with inner diameter of around 0.3 mm. For such small liquid samples, a fast and deep supercooling can be experimentally achieved while the liquid properties can still be considered unconfined and macroscopic.
Surface tension of supercooled water plays an important role by investigation of nucleation and growth of droplets and ice crystals in upper atmosphere. Based on the Laboratory results, the international standards for water [IAPWS R1-76(2014)] and seawater [IAPWS G14-19] surface tension could be recently extended into the supercooled region.
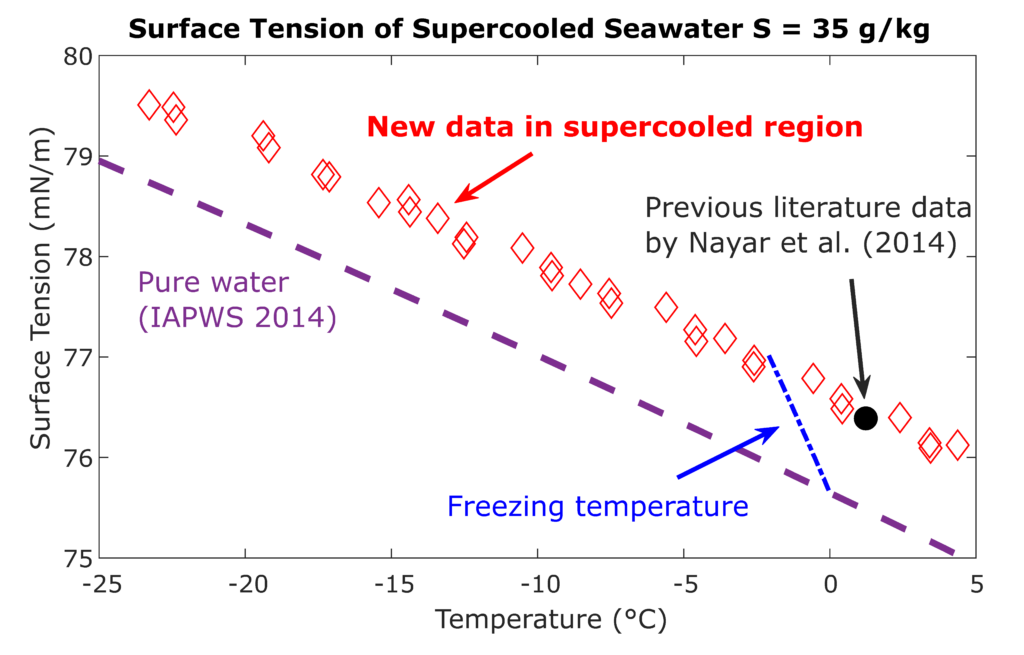
Fig. 1. Surface tension of seawater with a salinity of 35 g/kg below the freezing temperature
Modeling of gas hydrates
The Laboratory intensively collaborates with the groups from Ruhr-Universität Bochum (RUB) and Technische Universität Dresden (TUD) on the modeling of properties and phase equilibria of gas hydrates, i.e. solid mixtures of water and gases. In gas hydrates, the water forms crystal structure with cavities filled by gas molecules. Main scope of the joint research is a better description of CO2-rich systems relevant mostly in the design of new CCS/U (carbon capture and storage / utilization) technologies. The developed thermodynamic model is based on the van der Waals and Platteeuw approach combined with the highly accurate multiparameter equations of state for other phases. The LTP team is also partly involved in the extensions of the thermodynamic property package TREND developed at the Ruhr-Universität Bochum.
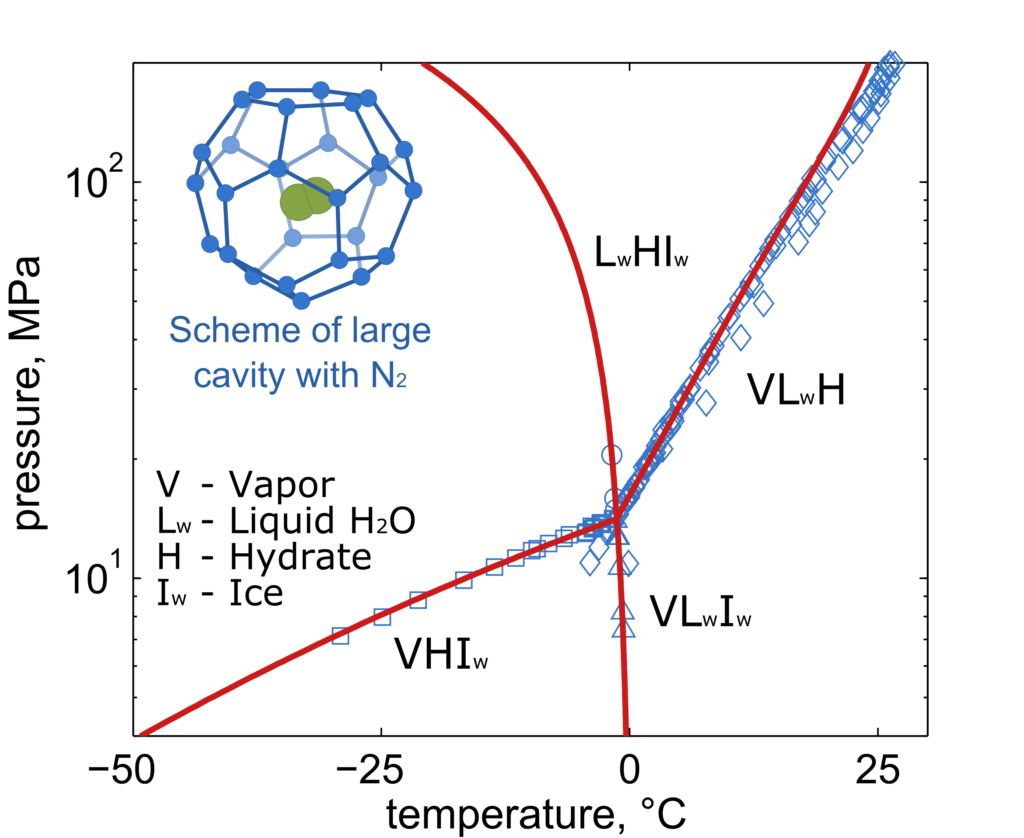
Fig. 2. Phase diagram of water + nitrogen mixture exhibiting formation of gas hydrates at low temperatures and high pressures; prediction of own model (red lines) compared to experimental data (blue symbols)
Prediction of vapor-liquid interfacial properties
Beside the experimental research, the surface tension and interfacial properties of vapor-liquid systems are modeled using the semi-empirical equations of state of SAFT-type combined with the density gradient theory. Properties of various systems including CO2 mixtures and refrigerants are modeled with good predictive ability. Correlation formulas for the surface tension of various homologous series of ionic liquids have been developed using the group contribution methods.
![Povrchové napětí směsi CO2 a n-butanu vypočtené pomocí stavové rovnice PCP-SAFT a gradientní teorie hustoty vs. experimentální data od Hsu a kol. [J. Chem. Eng. Data 30 (1985) 485]](https://www.it.cas.cz/wp-content/uploads/2020/12/Fig4_surften_CO2_C4-1024x772.png)
Fig. 3. Surface tension of CO2 + n-butane predicted by PCP-SAFT equation of state and density gradient theory compared to experimental data by Hsu et al. [J. Chem. Eng. Data 30 (1985) 485]
Instruments and apparatuses:
- Unique apparatus for measurement of the surface tension of supercooled aqueous systems at temperatures from -30 to 50 °C
- Vibrating tube densimeter Anton Paar DMA 5000 M for atmospheric measurements of liquid density with resolution of 0.001 kg/m3 at temperatures from 0 to 100 °C (standard uncertainty of measure density is typically around 0.03 kg/m3)
- High-pressure vibrating tube cell Anton Paar DMA HP operating at temperatures from -10 to +200 °C and pressures up to 70 MPa with accuracy of 0.1 kg/m3 (http://www.anton-paar.com)
- Tensiometer Krüss K100 Mk2 for measurement of surface tension and density at barometric pressure and temperatures from -10 to 90 °C (http://www.kruss.de)
- Stabinger viscometer Anton Paar SVM 3001 allowing measurement of viscosity of up to 30,000 mm2/s and density up to 3,000 kg/m3 (http://www.anton-paar.com)
Supporting equipment:
- Analytical balances Mettler-Toledo with resolution 0.0001 g in the range of up to 320 g (https://www.mt.com)
- Coulometric Karl-Fischer titrator Mettler-Toledo C30 for the measurement of low water content (under 50 ppm) in chemicals (https://www.mt.com)
- Resistance thermometry bridges ASL CRT5000 and ASL F700 with accuracy ± 10 mK and ± 27 mK, respectively (http://www.aslltd.co.uk)
- Set of data acquisition units Keysight (Agilent, HP) 34970A with multiplexer cards (http://www.keysight.com)
- Set of thermostatic baths Lauda for temperature control from -70 °C to 150 °C (https://www.lauda.de/en)
Bachelor and Master thesis topics:
Czech Technical University in Prague, Faculty of Nuclear Sciences and Physical Engineering, Department of Mathematics
Name: Modelling of thermophysical properties of liquids and gases with the PC-SAFT equation of state
Supervisors: Dr. Václav Vinš and David Celný MSc.
Keywords: Nonlinear regression, thermodynamics, equation of state, phase equilibrium, refrigeration
Description: Development of modern engineering applications requires the accurate knowledge of thermophysical properties of working fluids. In areas such as energy industry, cooling and refrigeration, natural gas industry, or development of new CCS technologies, it is necessary to know properties such as density, vapor pressure, heat capacity or phase behavior over wide temperature, pressure and composition ranges. In these areas, the modern semi-empirical equations of state like PC-SAFT start to find their use. With relatively good accuracy, these equations provide predictions of required properties for large variety of substances ranging from simple gases (argon, nitrogen, CO2), hydrocarbons to multicomponent mixtures. The topic of the work fits into long-term research activities of the Laboratory of Thermophysical Properties at IT CAS. The offered topic focuses primarily on the development and implementation of non-linear algorithms for the optimization of the PC-SAFT fluid parameters such as Levenberg-Marquardt algorithm. Additional tasks include implementation of the model for the Helmholtz energy of an ideal gas and of a more sophisticated phase equilibrium algorithm for multicomponent mixtures. The developed model will be validated on the experimental data. The researches from IT CAS will help with the physical background of the problem. The obtained results will be directly used in the experimental research of the Laboratory. The candidate should have positive attitude to technical computations and programming.
Dissertation topics:
Technical University of Liberec, Faculty of Mechanical Engineering, Department of Energy Devices
Name: Properties of water-based heat transfer liquids in the supercooled metastable state
Supervisor: Dr. Václav Vinš
Keywords: cooling, experiment, metastable state, surface tension, equation of state, thermophysical properties, water
Description: The topic of dissertation covers the experimental and theoretical research of thermophysical properties of heat transfer liquids based on water. The investigated systems include aqueous mixtures with alcohols (e.g., ethylene glycol, glycerol or methanol) and salts (e.g., NaCl, CaCl2). The properties of selected mixtures will be studied in the thermodynamic equilibrium state and also under the metastable supercooled state below the freezing point temperature. The experimental part will contain the measurement of density and surface tension depending on temperature and composition. Theoretical part of the work will focus on development of correlations applicable in the practical design of cooling systems and on the modeling of interfacial properties and nucleation using the PC-SAFT equation of state in combination with the density gradient theory. The candidate should have positive attitude to experimental work and also to technical calculation and programming.
Collaborating institutions:
- Ruhr-Universität Bochum, Germany - Thermodynamics department of Prof. Roland Span
- Technische Universität Dresden, Germany - Department of Thermal Power Machinery and Plants led by Dr. Andreas Jäger
- Technische Universität Chemnitz, Germany - Professorship Applied Thermodynamic of Prof. Markus Richter
- Technical University of Liberec - Department of Power Engineering Equipment
- University of Chemistry and Technology, Prague - Laboratory of Applied Thermodynamics