Laboratory of Phase Transitions
|
Department: Department D 2 - Thermodynamics Head: Ing. Jan Hrubý, CSc. |
|||
Laboratory of Phase Transitions (LPT) focuses on experimental and theoretical research of phase transitions when in a bulk phase in thermodynamically metastable state start to form and subsequently grow clusters of a new phase. The formation of new phase, otherwise known as nucleation, happens for example by the generation of droplets in the supersaturated steam, the formation of bubbles in the superheated liquid, or the crystallization of solid phase in the supercooled liquid. LPT addresses the environmental issues, e.g., the formation of aerosol, water droplets, and ice crystals in atmosphere, together with the industrial applications such as condensation of droplets in steam turbines, cavitation in pumps, and formation of ice crystals in fuel cells. LPT investigates thermophysical properties both under the thermodynamically stable and metastable conditions needed for an accurate description of the phase transitions. The LPT members are involved in activities of the Czech National Committee for the Properties of Water and Steam (CZNCPWS) and the international association IAPWS, where they within an international research team investigate thermophysical properties, phase equilibria and phase transitions of aqueous systems, e.g., properties of supercooled liquid water at temperatures down to –30 °C or phase equilibria of aqueous systems with gas hydrates.
Fig. 1 Tree branches covered with a continuous layer of ice, which emerged after the rain of supercooled water | |||
Ing. Jan Hrubý, CSc.
Ing. Václav Vinš, Ph.D.
Ing. Miroslav Čenský, Ph.D.
Ing. Aleš Blahut, Ph.D.
MSc. Mykola Lukianov, Ph.D.
Ing. Tetiana Lukianova
Density of supercooled water
Density apparatus is designed for measurement of density of supercooled aqueous liquids, e.g., supercooled ultrapure water, sea water, or heavy water (D2O), at a constant pressure. The apparatus allows measuring isothermal compressibility for pressures up to 200 MPa (1974 atmospheres) and temperatures down to –40 °C. Main goal of the experiment is to clarify and describe the anomalies of supercooled water at low temperatures and high pressures.
 a) a) |
 b) b) |
Fig. 1 Apparatus for measurement of density of supercooled liquids at high pressures
a) High pressure cell inside a safety containment (red cylinder) and the temperature control setup
b) System for regulation of pressure of nitrogen up to 200 MPa
Density of supercooled water is determined from the comparative measurement inside two fused silica capillary tubes with constant inner diameters of about 0.3 mm. Upper parts of the vertical capillary tubes are installed inside a stainless steel (SS) high-pressure cell equipped with a set of sapphire windows allowing observation of the capillary tubes by an objective and a digital camera. The high-pressure optical cell is kept at a constant temperature close to ambient.
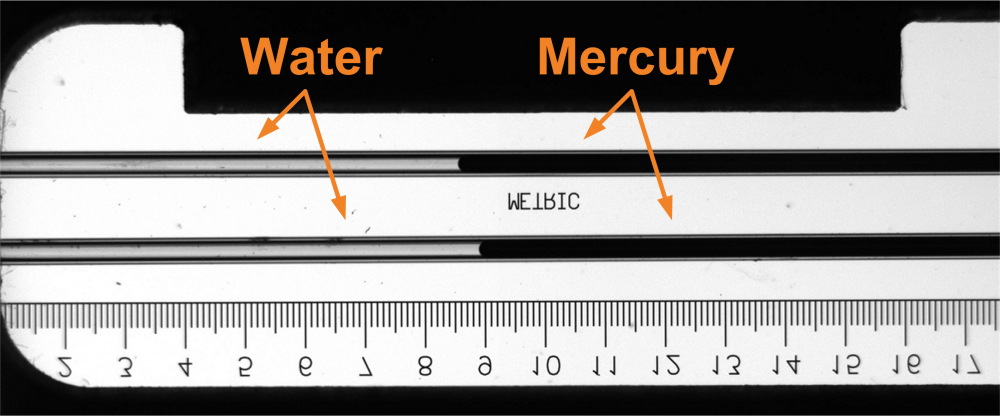
Fig. 2 Image of the capillary tubes inside the high-pressure cell equipped with a set of sapphire windows taken by the digital camera (the image is rotated by 90°)
Lower parts of the capillary tubes are placed inside a protective SS tube filled with a low-freezing heat transfer (ionic) liquid. The pressure inside the optical cell and the protective tube is induced by a gaseous nitrogen pressurized by a high-pressure compressor. A mercury plug long a few millimeters is used to separate the water sample from nitrogen to prevent its dissolution and bubble formation upon pressure reduction.

Fig. 3 The high-pressure optical cell together with the safety containment in open position (red cylinder below)
The lower ends of the capillary tubes are located within the heat exchanger where the density of the liquid sample changes due to the varying temperature. The heat exchanger is alternatively connected to a cooling circulation thermostat delivering ethanol at desired temperature (e.g., –15 °C) and to another circulation thermostat providing ethanol at a reference temperature (e.g., + 20 °C). The heat exchanger has a low thermal capacity allowing that the temperature stabilizes to about ± 0.02 °C in several minutes. The advantage of using two capillaries, one long and the other short, is that the effect of temperature transition zone is eliminated by taking the difference of heights of liquid columns in both capillary tubes.
Surface tension of supercooled water
Surface tension apparatus is designed for measurement of surface tension of aqueous liquids both in the thermodynamic equilibrium and in the metastable state. The operating temperatures lie between –30 °C and +50 °C. Main aim of the measurements is to collect new experimental data under the metastable conditions in order to clarify some of the anomalies of the supercooled water, e.g., the second inflection point in the surface tension of water detected by previous experiments and some molecular simulations. Surface tension of supercooled water plays an important role by investigation of nucleation and growth of droplets and ice crystals in upper atmosphere and also by many technical applications, e.g., freezing of aircrafts in clouds formed by the supercooled droplets or freezing of wind turbines blades.

Fig. 4 Fused silica capillary tube with inner diameter around 0.3 mm placed inside the temperature-controlled glass chamber
The surface tension of supercooled water is measured inside fused silica capillary tubes with small inner diameter (around 0.3 mm). The apparatus allows measurement with two different methods. In the main method based on the capillary rise technique, kthe liquid column in a vertical capillary tube elevates to a certain height (several centimeters) given by the surface tension and the density of liquid, or eventually the hydrostatic pressure of the liquid column. The surface tension can be determined from the height of the liquid column, the inner diameter of the capillary tube, and the liquid density. On the apparatus for supercooled liquids, the upper part of the elevated liquid column, located inside the temperature-controlled chamber, can be rapidly cooled down for a short time such that the solidification of liquid sample is prevented. The surface tension increases with decreasing temperature, thus the liquid column elevates to a higher position. The temperature dependence of the surface tension can be obtained from the measured difference of height corresponding to the given temperature change.
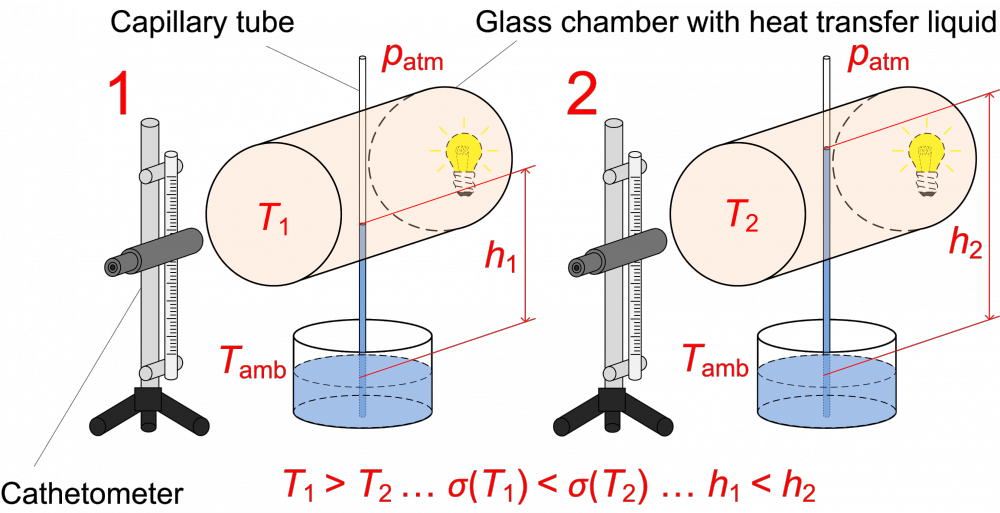
Fig. 5 Principle of the capillary rise method for the measurement of surface tension
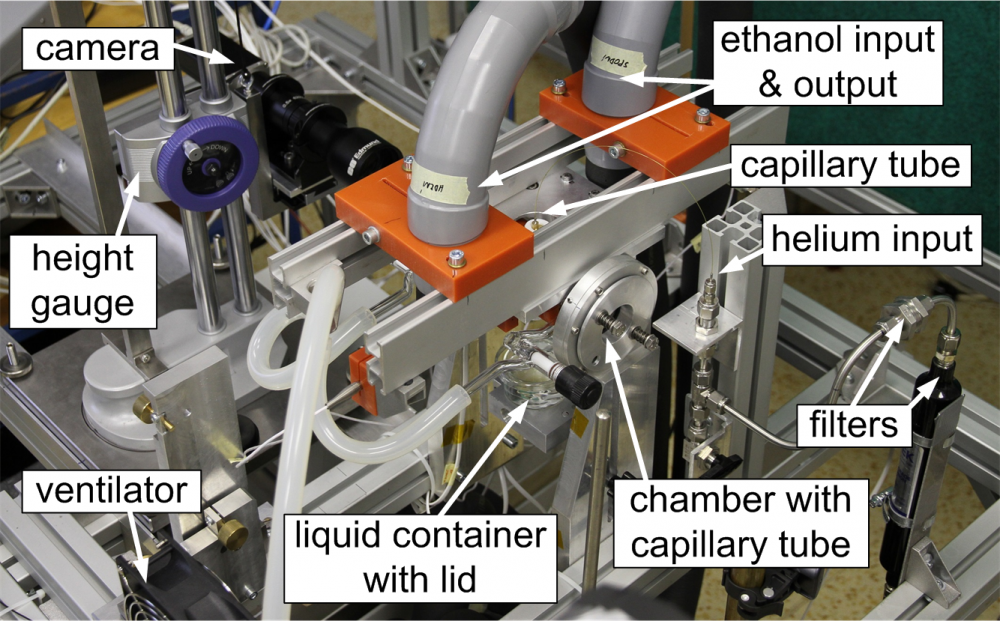
Fig. 6 Main components of the capillary rise apparatus
The second method for the measurement of surface tension of supercooled liquids uses the capillary tube in a horizontal position. Liquid sample of a length of 2 to 3 cm is sucked inside a horizontal capillary tube with one precisely grinded planar end. The grinded end of the capillary tube is left open to ambient. It is observed with sensitive optics. The other end of the capillary tube is connected to a system with inert gas allowing precise adjustment of a low counterpressure in order of several hundreds of Pascal. With increasing counterpressure of inert gas, the liquid meniscus at the open end of the capillary tube gradually changes from concave (inside the capillary tube) to planar and subsequently convex (meniscus outside of the capillary tube). In case of the exactly planar outer meniscus, the counterpressure is in equilibrium with surface forces at the second meniscus inside the capillary tube. Consequently, the surface tension at the inner meniscus can be determined from the measured counterpressure of inert gas and the inner diameter of the capillary tube.
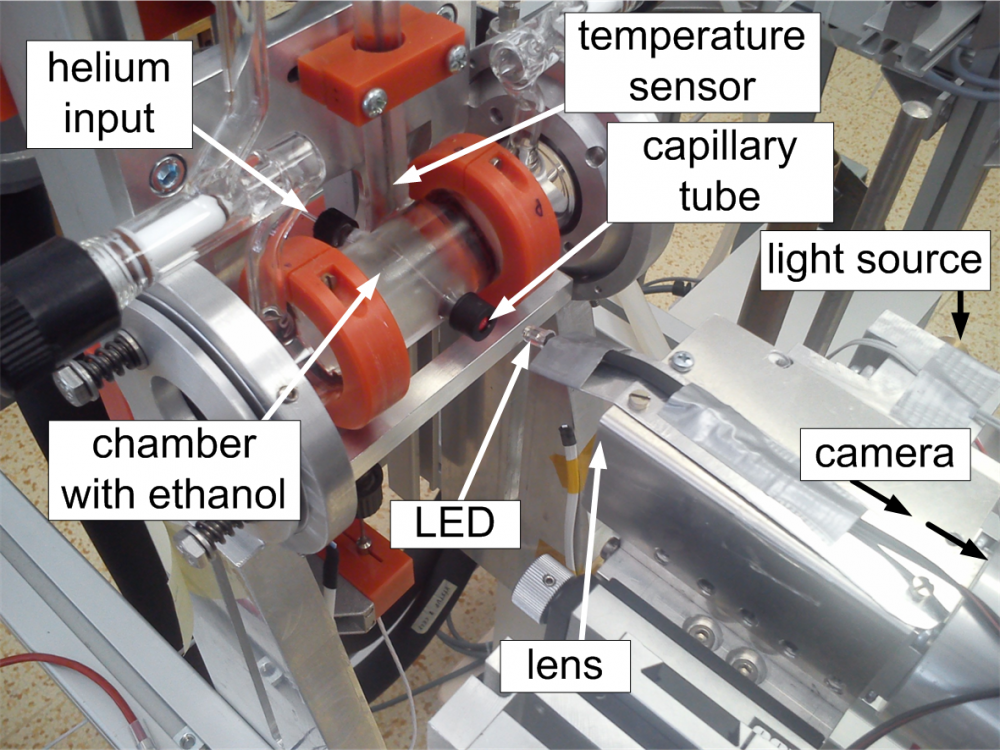
Fig. 7 The horizontal capillary tube installed inside the temperature-controlled chamber with the heat transfer liquid (mostly ethanol)
Nucleation of droplets in CCS systems
A new experimental apparatus for investigation of nucleation of droplets in systems relevant for CCS (carbon capture and storage) is currently developed at the Laboratory of Phase Transition Kinetics. Main goal of this research is to obtain new experimental data for the nucleation of droplets in supersaturated vapor of mixtures important in the design of new CCS technologies, e.g., the CO2 rich systems with nitrogen, water, methane, and argon. The nucleation of droplets, i.e. the inception of phase transition between the supersaturated vapor and the liquid, is studied with the help of an abrupt pressure drop inside a stainless steel expansion chamber with inner volume of around 200 cm3. The expansion chamber is equipped with a set of optical windows allowing detection of the nucleation conditions with the use of precise optics (interferometer). The experiment will allow detecting the nucleation of droplets at pressures from 2 to 140 bar and temperatures between –20 °C and +150 °C.
More information about this research is given on website of the international project CCSphase.
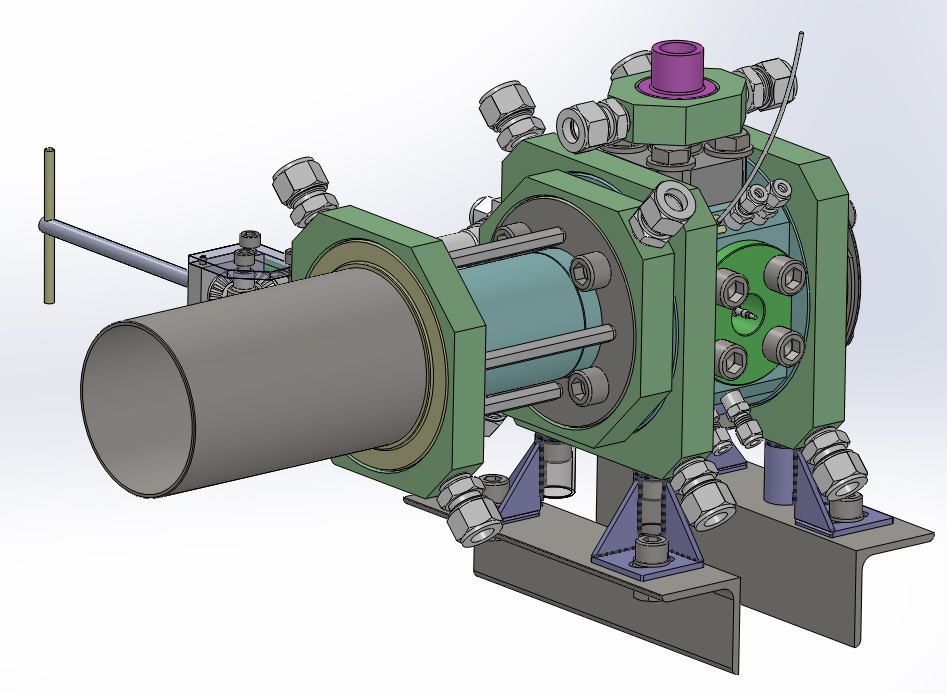
Fig. 8 3D design of the expansion chamber
In the Laboratory of Phase Transition Kinetics, three main experimental apparatuses are currently being developed:
- Apparatus for measurement of the density of supercooled aqueous liquids at temperatures down to –40 °C and pressures up to 200 MPa
- Apparatus for measurement of the surface tension of supercooled water down to –30 °C
- Expansion chamber for investigation of the nucleation of droplets in the CCS relevant systems
Other important equipment of the laboratory
- Two independent systems for regulation of low temperatures down to –70 °C consisting of thermostatic baths Lauda Proline (http://www.lauda.de)
- High-temperature thermostatic bath Lauda USH 400 reaching temperatures up to + 400 °C (http://www.lauda.de)
- Analytical balances Mettler-Toledo XSE205DU with resolution 0.01 / 0.1 mg in the ranges 81 / 220 g (http://us.mt.com)
- Reverse osmosis Rowapur for preparation of pure water (http://www.watrex.cz)
- Neptune Analytical for preparation of ultrapure water with resistivity 18.2 MΩ.cm and TOC < 5 ppb (http://www.purite.com)
- Resistance thermometry bridges ASL CTR6500 and ASL F500 with accuracy ± 0.25 mK and ± 5.0 mK, respectively (http://www.aslltd.co.uk )
- Digital height gauge Mitutoyo HD-A Digimatic 600 mm with cathetometer Titan TC-II (http://www.mitutoyo.cz, http://www.titantoolsupply.com)
- Digital monochromatic camera with high resolution Basler pilot piA2400-12gm (http://www.baslerweb.com)
- Set of data acquisition units Keysight (Agilent, HP) 34970A with multiplexer cards (http://www.keysight.com)
- Set of precise pressure transducers for measurement of pressures from several Pascals to 200 MPa (Furness Controls, GE Druck, Keller, Paroscientific)
- Two instruments operated in collaboration with the Laboratory of Thermophysical Properties of Fluids.
- Densitometer with vibrating tube Anton Paar DMA 5000 M with high-pressure cell DMA HP reaching accuracy of ± 0.0001 g/cm3 at operating temperatures from –10 to +200 °C and pressures up to 70 MPa (http://www.anton-paar.com)
- Tensiometer Krüss K100 Mk2 for measurement of surface tension and density of liquids (http://www.kruss.de)
Collaborating institutions:
- Czech Technical University in Prague, Faculty of Mechanical Engineering – Department of Energy Engineering (M. Kolovratník)
- Ruhr-Universität Bochum, Germany – Thermodynamics department of Prof. R. Span
- SINTEF Energy Research, Norway – Gas Technology department (S. Løvseth, J. Jakobsen)
- Technical University Eindhoven, the Netherlands – Energy Group of Prof. D. Smeulders
- Technische Universität Dresden, Germany – Chair of Technical Thermodynamics of Prof. C. Breitkopf
- Institute of Chemical Process Fundamentals of the CAS, v. v. i. – (V. Ždímal, M. Lísal, M. Bendová

 Fig. 1
Fig. 1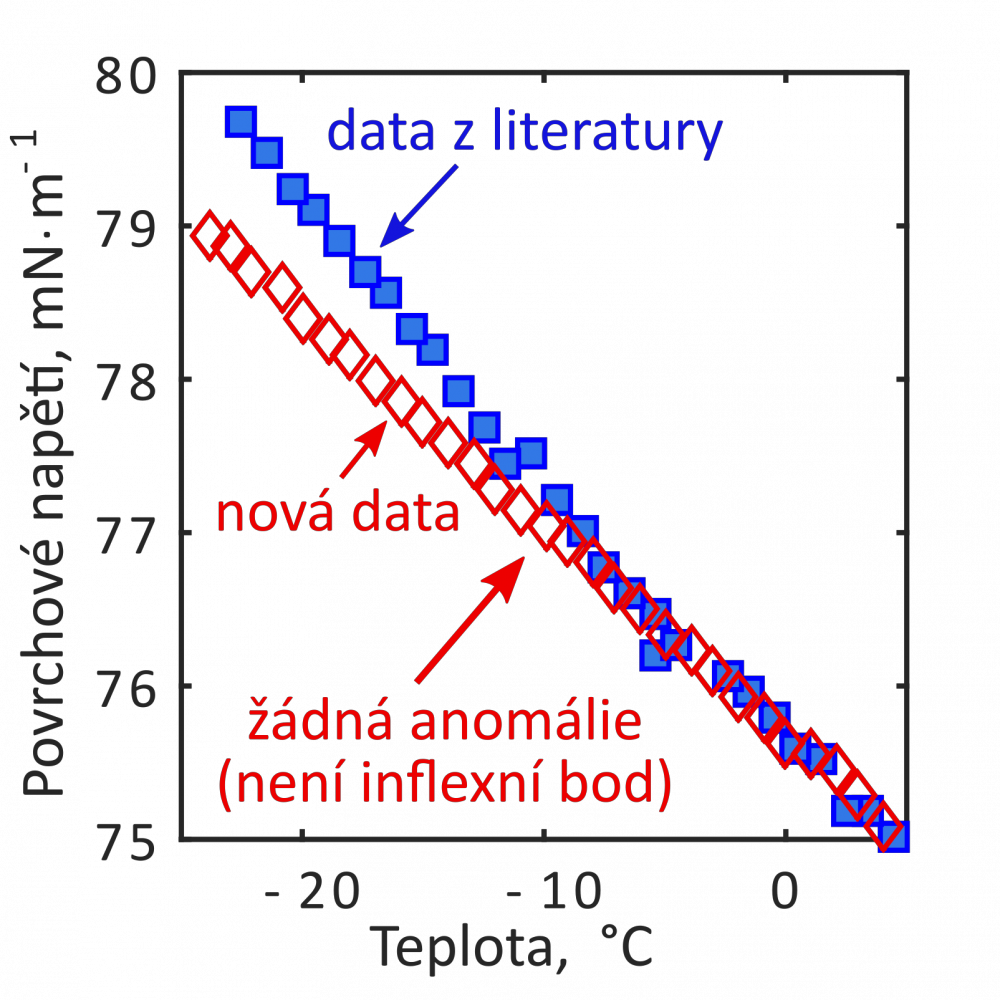 Fig. 2
Fig. 2